Research
Overview
The Center for Closing the Carbon Cycle (4C) is advancing the foundational science and defining key integration parameters for synergistic CO2 capture and conversion, or reactive capture of CO2 (RCC). By linking the study of CO2 sorbent properties with catalysts for functionalization, 4C is developing integrated CO2 capture and conversion systems that work cooperatively to achieve higher product selectivity and overall efficiencies. 4C will establish guidelines for CO2 capture from various dilute and dirty streams and define how captured CO2 can most effectively be utilized, leading to durable, efficient, and direct pathways from CO2 source-to-product.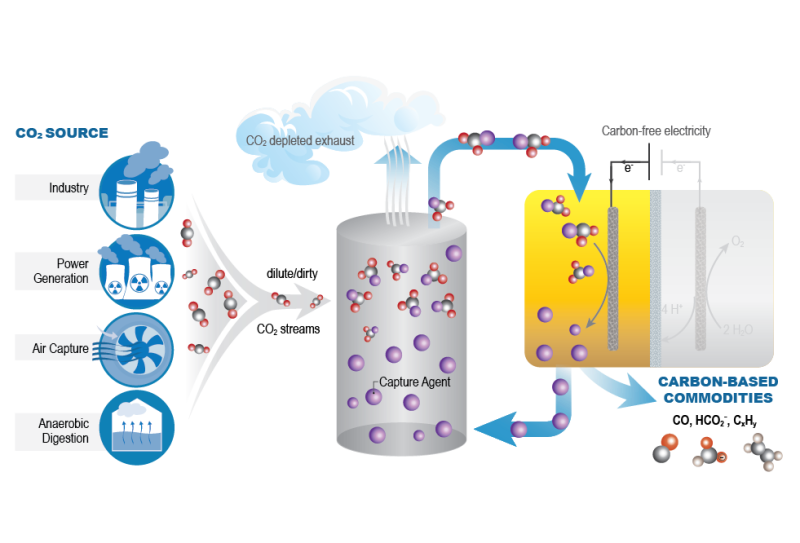
Chemistry of CO2 Capture
Most research on CO2 capture agents has focused on sorbents suitable for capturing CO2 from point sources, such as flue gas, combined with releasing the captured CO2 using a thermal or pressure cycle. These applications have constrained the search for CO2 sorbents. 4C is exploring the chemical space for CO2 sorbents and establish correlations between electronic and steric structure and CO2 binding properties and tolerance to oxygen, water, and other common contaminants among different chemical classes. 4C is also exploring the impact of the surrounding microenvironment on CO2 sorption, including the effect of solvent, electrolyte, and additives.
Sorbents compatible with homogeneous and heterogeneous catalysts are being explored, including: 1) soluble organic species and 2) functionalized solvents, which include ionic liquid and eutectic-based solvents. The chemical sorbent space is explored through a combination of computational screening, chemistry-guided high throughput experimentation, and machine learning/AI methods coupled with validation by scaled-up experimentation. Neutron scattering provides a link between macroscopic CO2 binding and picosecond dynamic simulations, and provides crucial insights to speciation and structural reorganization at atomic length scales that overlap with computational approaches. This information in turn is used by 4C to develop improved models for CO2 binding, solvation energies, and understanding the effect of microenvironment. The result will be a comprehensive description of the chemistry of CO2 absorption through spectroscopic characterization of the microenvironment and theoretical modeling and AI.
Co-Designing Sorbents for Reduction
4C is determining parameters for CO2 sorbents and their microenvironments for synergistic catalytic functionalization. To complement the efforts on catalyst design, 4C is 1) verifying the electrochemical stability (reductive window) and conductivity of the sorbents with ideal CO2 capture properties and 2) understanding microenvironment that can be tuned via solvent composition (electrolyte or co-solvents additives) to improve mass and ion transport without reducing the CO2 capture capacity. The computational modeling and AI methods and in situ spectroscopic measurements links bulk properties and interfacial reactivity. Known catalysts for CO2 reduction are being tested for with the capture agents developed in 4C to determine general compatibility guidelines.
Binding CO2 is expected to introduce a dipole into this otherwise inert and linear molecule, which will provide kinetic activation for subsequent reduction. 4C is capitalizing on this effect by designing sorbents that are tailored for pre-activation and subsequent functionalization. For this purpose, a balance of capture performance vs subsequent stability would have to be achieved to optimize overall performance for capture and conversion. 4C is also exploring cooperative functionality in sorbents to activate and position CO2 for further functionalization.
Catalyst Design
Our understanding of mechanisms and guiding concepts that operate in electrochemical CO2 Reduction (CO2R) include canonical reaction paths and thermodynamic descriptors for reactivity and selectivity. Recent studies have illuminated the effect of catalyst composition, specific ions, dielectric constant, and pH on activation barriers, reaction selectivity, and product selectivity. We currently do not have a clear understanding of how these principles translate to RCC, as captured CO2 adducts are intrinsically a different substrate for catalytic conversion with a broad range of molecular diversity.
To aid the coevolution of catalysts and capture agents, 4C is using simulations to prescreen potential catalysts for accessible activation barriers to likely transformations, as well as whether the RCC microenvironment can promote catalysis. Molecular descriptors of capture agents that are effective predictors of activation barriers and inhibition are identified and tested experimentally through electrochemical kinetic and mechanistic studies of predicted catalytic intermediates.
4C is defining translatable knowledge about CO2R to RCC and identify unique opportunities presented by RCC by tailoring sorbents to enable new catalytic transformations. The strength in our approach is bridging knowledge between the fields of molecular catalysis, heterogeneous catalysis, and functionalized solvents to develop efficient and selective homogeneous, heterogeneous, and hybrid catalysts for the valorization of captured CO2 to C-based fuels and commodities. 4C is addressing supply chain security by understanding catalytic mechanisms and tailoring reactivity with non-critical and non-rare earth materials.
RCC Integration
The optimal sorbent properties for CO2 capture and direct functionalization will ultimately depend on the dilute feedstock stream and subsequent catalytic application. RCC offers the opportunity to tune the physical and reactivity properties of the captured CO2 substrate to access a broader scope of chemical transformations compared to traditional CO2 chemistry. Moreover, the dynamics between CO2, the capture agent, supporting electrolyte, applied potential, and solvent create alternative catalyst microenvironments. These microenvironments differ significantly from the bulk electrolyte in composition, pH and local temperatures, and they will determine the observed rates of transfer and dissipation for mass and energy during operation. 4C is working to understand these dynamics to inform the solvent and catalyst discovery efforts with design criteria from real world operating conditions. We are leveraging a non-dimensional analysis approach to establish descriptors that can be used to co-design integrated CO2 capture and conversion systems. By studying the mechanisms and kinetics of both CO2 capture and conversion together, 4C is able to match CO2 capture and conversion timescales via co-design of sorbents and catalysts. 4C is tackling the challenges of energy affordability and reliablity by determining degradative processes and how to improve stability of the systems.
© Copyright Center for Closing the Carbon Cycle (4C). All Rights Reserved.
